Everyday examples of Crystallization in action:
Salt
Salt can be obtained from sea water by evaporating water causing salt to crystallize out.
Pharmaceuticals
Crystiallization is often found in the final separation and purification steps for synthesis in the pharmaceutic industry, where high levels of purity are needed
Film photography
Old photographic films made use of crystallization to precipitate silver halide, the light sensitive component that made photography possible
Industrial catalysts
A large number of industrial heterogeneous catalysts become less effective overtime. They can be recovered by dissolving in suitable solvents followed by recrystallization.
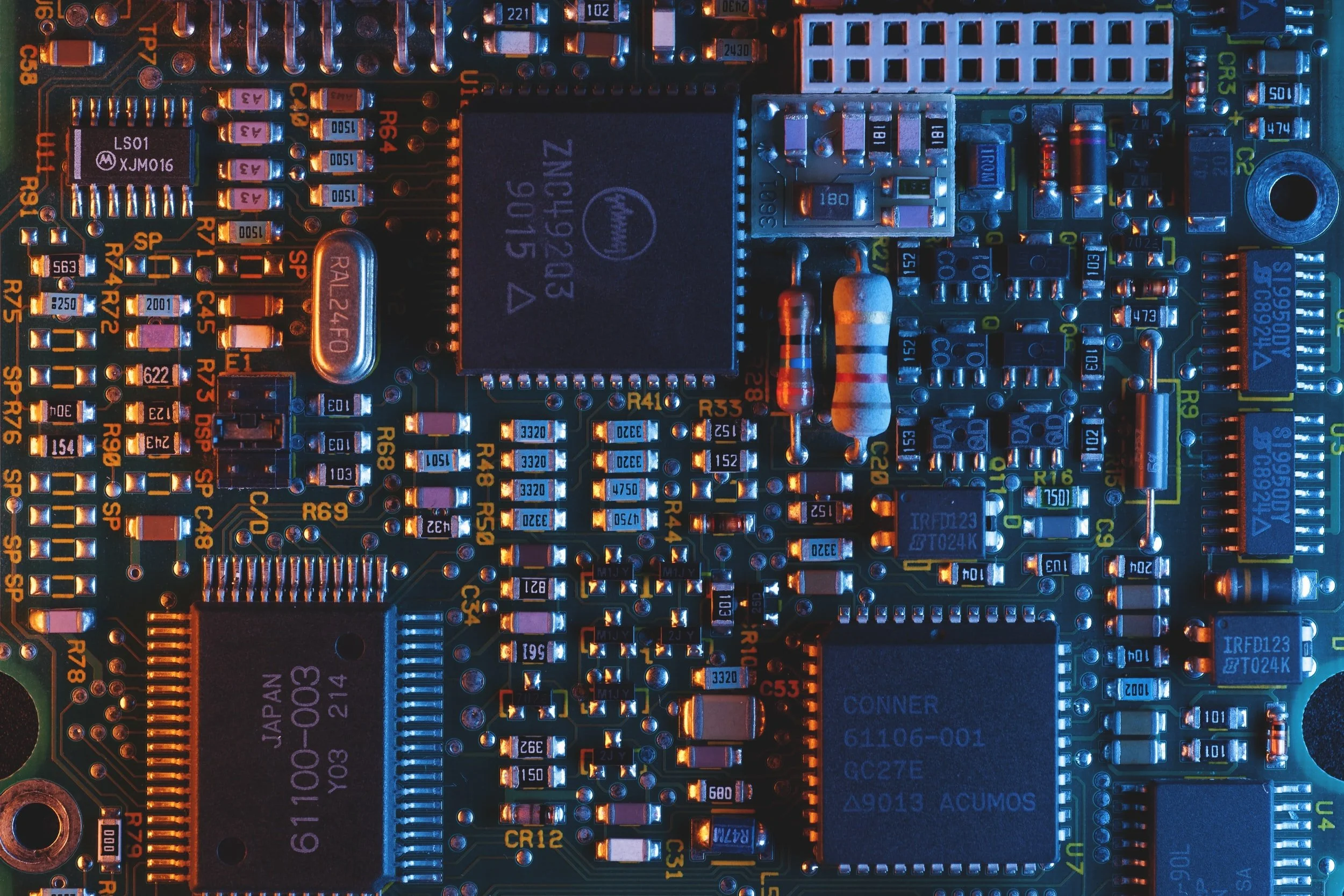
Electronics
Semiconductors, which are the building blocks of all our electronic devices, need very high purity. This is achieved by melting the metal at one edge, moving the molten region across along with impurities, and letting purified part to recrystalise.
Sugar
Sugar processing industry employs crystallization as its final refining process. Pure sucrose crystallizes out of sugar syrup or liquor leaving impurities in the syrup.